Adverse Incident Reports Show 966 Deaths Following Vaccination for COVID-19
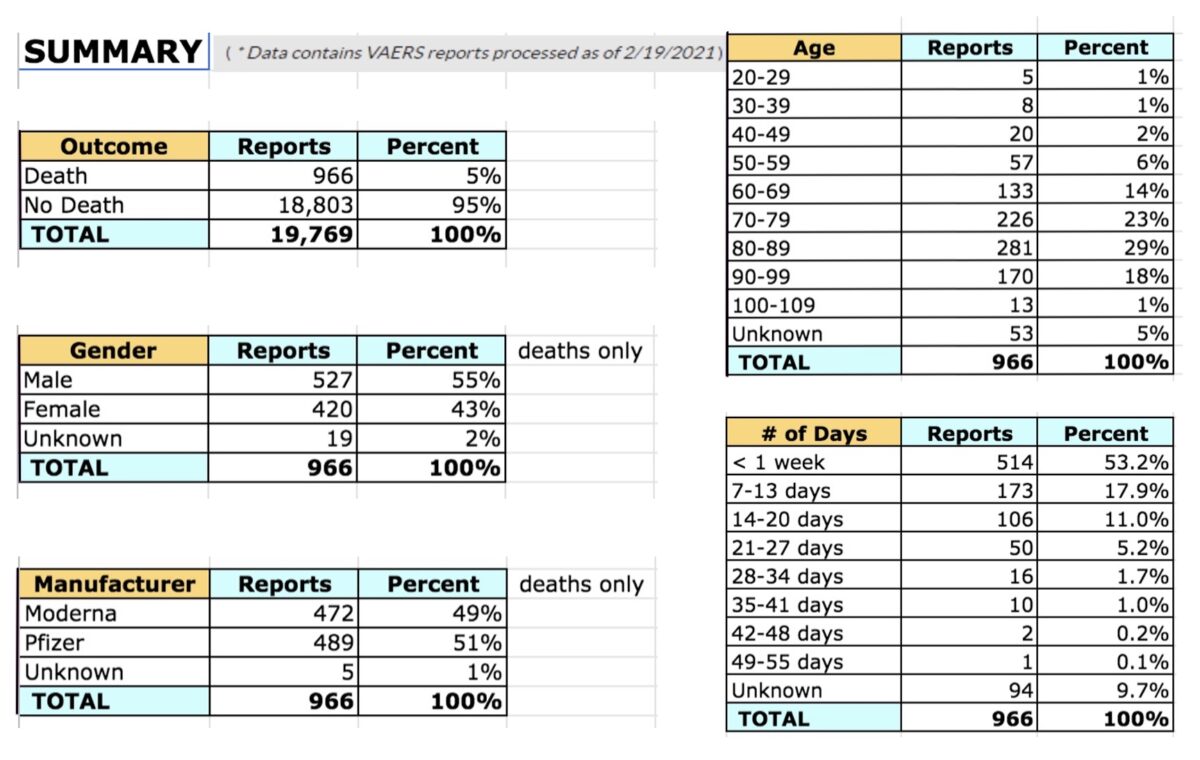
According to adverse incident reports collected by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) 966 individuals have died after having received an mRNA vaccine for COVID-19.
Between Dec. 14 and Feb. 19, 19,769 reports were made to the Vaccine Adverse Events Reporting System (VAERS) following immunizations with either the Moderna or Pfizer BioNTech mRNA vaccines (the only two vaccines given during the time period assessed). At this time, VAERS data is not available after Feb. 19.
The 966 deaths represent 5 percent of the total number of adverse events reports. Of those who died, 86, (8.9 percent) died on the same day they got the shot. An additional 129, (13.4 percent) died within one day. An additional 97 died within 2 days, and 61 within 3 days.
A total of 514 (53.2 percent) died within a week. 173 died within 7-13 days. 106 within 14-20 days.
85 percent of deaths occurred in individuals over 60; below 60 there were five deaths among those aged 20-29; 8 aged 30-39; 20 aged 40-49; and 57 aged 50-59.
For detailed information drawn from the VAERS reports, see charts provided at the link at the end of this article.
Comparison With Influenza Vaccines
Neither of the mRNA vaccines are FDA approved, rather, they have Emergency Use Approval (EUA). They represent a departure from traditional vaccines in that they do not use any part of the suspected pathogen to stimulate the immune system, but rather, nucleoside messenger RNA.
Dr. Christian Perrone, head of Infectious Disease at Hopital de Garches in France, stated in a complaint filed in Europe:
"The first vaccines they are offering us are not vaccines. They are gene therapy products. They…inject nucleic acids that will cause our own cells to produce elements of the virus."
The death rate following COVID mRNA vaccination is much higher than that following influenza vaccination.
The CDC's data allows only a ballpark estimation of the rate of deaths following flu vaccination.
In the 2019-2020 influenza season the CDC reports that 51.8 percent of the U.S. population received a vaccine, which is approximately 170 million people.
VAERS reports that in the calendar year 2019 (not the 2019-2020 influenza season) there were 45 deaths following vaccination. To provide context, in 2018 VAERS reports 46 deaths, and in 2017 it reports 20 deaths.
The 45 deaths in 2019 are occurring at a rate of 0.0000265 percent, when calculated using the number of vaccines given in the 2019-2020 influenza season.
As of Feb. 19, 41,977,401 COVID vaccinations had been given with 966 deaths reported following vaccination, which is approximately a rate of .0023 percent.
The VAERS System
VAERS was put in place in 1990, to capture unforeseen reactions from vaccines.
VAERS is criticized both for the fact that anybody can submit a report, and for the fact that it catches only a fraction of the adverse incidents.
The VAERS website describes the system in this way:
"Established in 1990, the Vaccine Adverse Event Reporting System (VAERS) is a national early warning system to detect possible safety problems in U.S.-licensed vaccines. VAERS is co-managed by the Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA). VAERS accepts and analyzes reports of adverse events (possible side effects) after a person has received a vaccination. Anyone can report an adverse event to VAERS. Healthcare professionals are required to report certain adverse events and vaccine manufacturers are required to report all adverse events that come to their attention.
"VAERS is a passive reporting system, meaning it relies on individuals to send in reports of their experiences to CDC and FDA. VAERS is not designed to determine if a vaccine caused a health problem, but is especially useful for detecting unusual or unexpected patterns of adverse event reporting that might indicate a possible safety problem with a vaccine. This way, VAERS can provide CDC and FDA with valuable information that additional work and evaluation is necessary to further assess a possible safety concern."
Without a medical diagnosis or autopsy, the report of an adverse incident following a vaccination is not proof that the vaccination caused any particular symptoms.
In a reply to The Epoch Times, about the VAERS death report, Steven Danehy, Director of Global Media Relations for Pfizer, wrote:
"To date, millions of people have been vaccinated with our vaccine. Serious adverse events, including deaths that are unrelated to the vaccine, are unfortunately likely to occur at a similar rate as they would in the general population."
Moderna has not responded to requests for comment.
The VAERS database is dense with information and can be difficult for some users to follow. The Epoch Times has extracted its data as clearly as possible in charts provided in the link below.
At the link below are charts containing: on the tab "All Deaths Readable" descriptions of what happened to the patients—effects they experienced as reported by health care workers and/or relatives, or other witnesses; VAERS ID numbers (used to look up a complete file on the VAERS database); vaccination type; manufacturer; vaccination name; date received; age, gender and state of each recipient; as well as medical history; and other medications patients were taking.
No comments:
Post a Comment